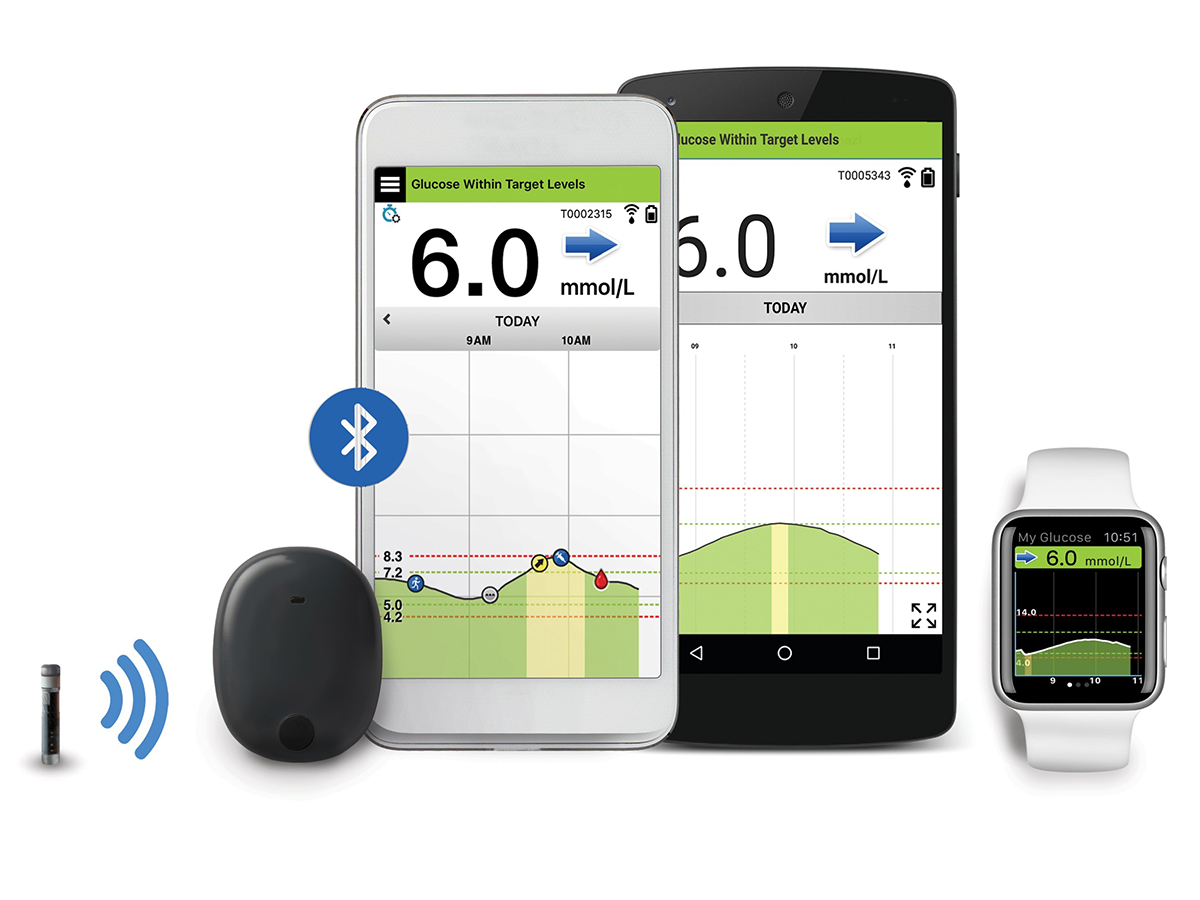
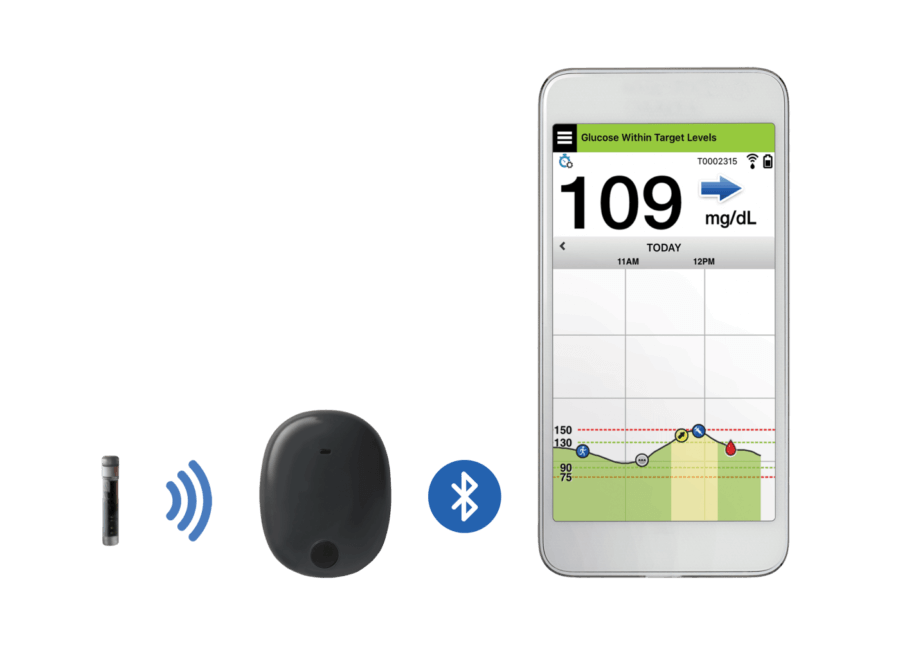
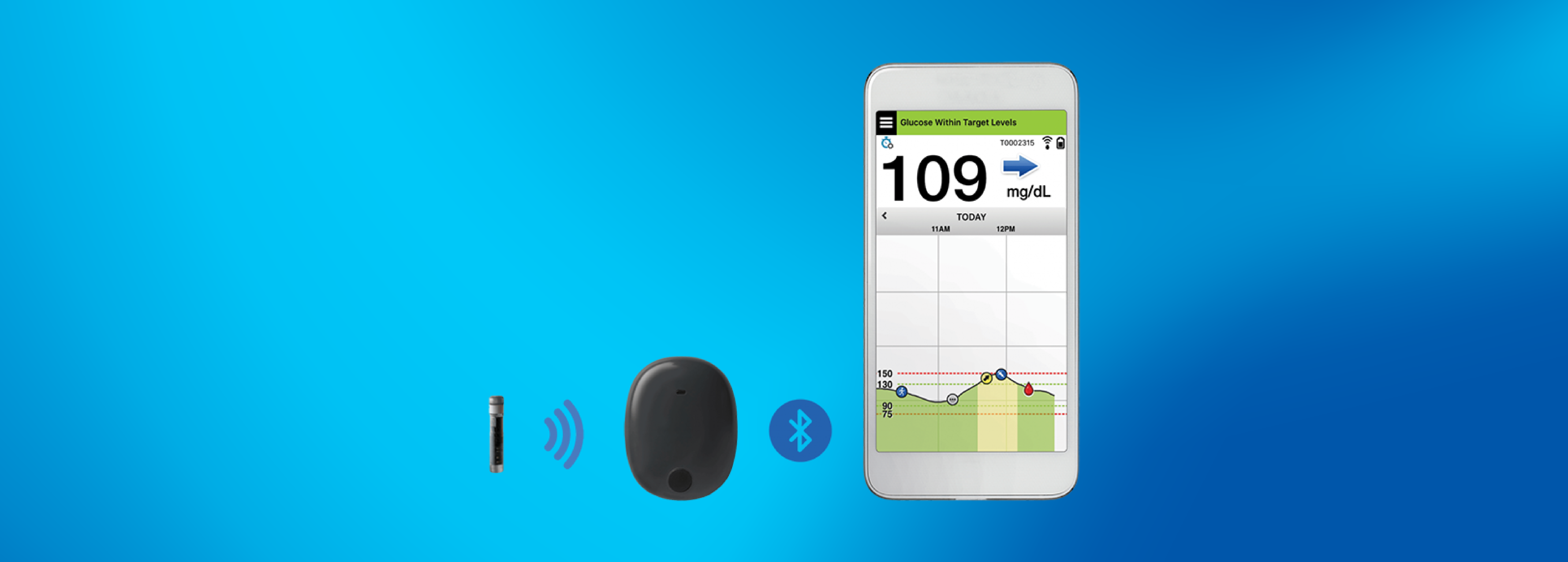
Continuous Glucose Monitors: FDA Grants Pediatric Indication to Medtronic Device, Approves Senseonics System | RAPS

Senseonics gets FDA approval for non-adjunctive indication for Eversense 90-day CGM system - NS Medical Devices

FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News

FDA approves Senseonics' application to sell implantable glucose monitor - Washington Business Journal