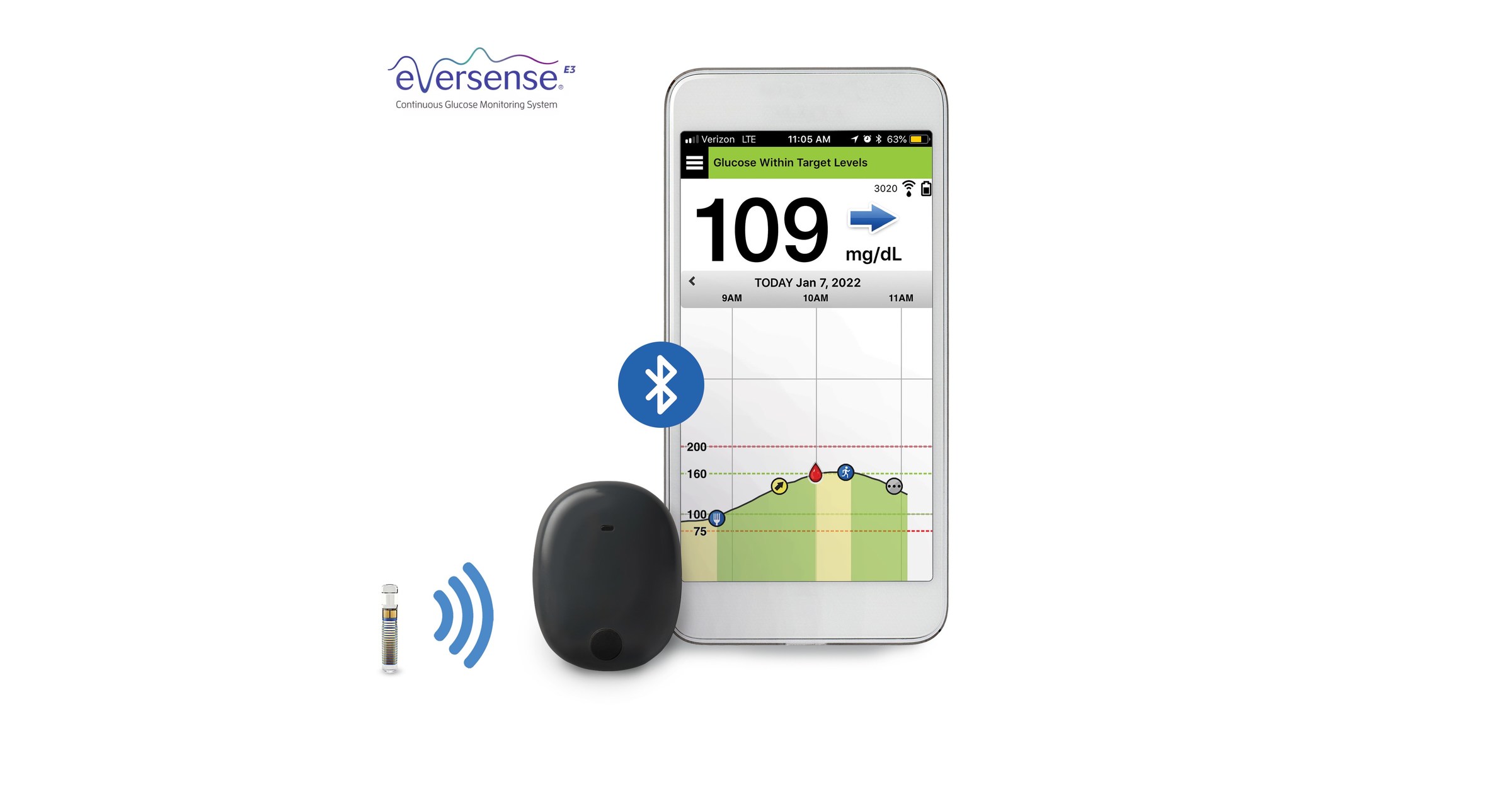
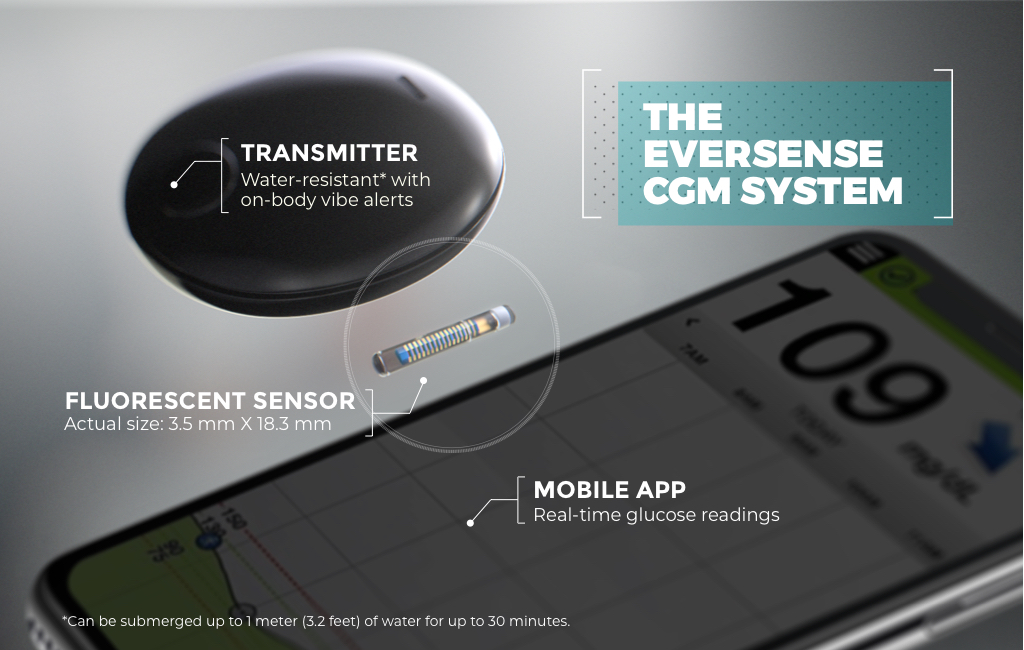
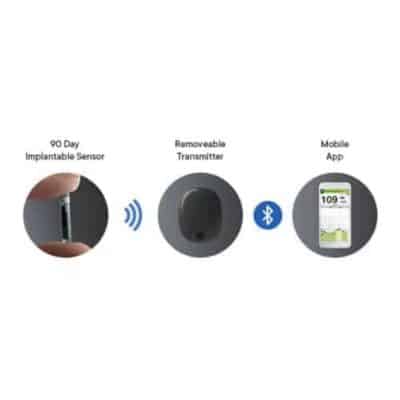
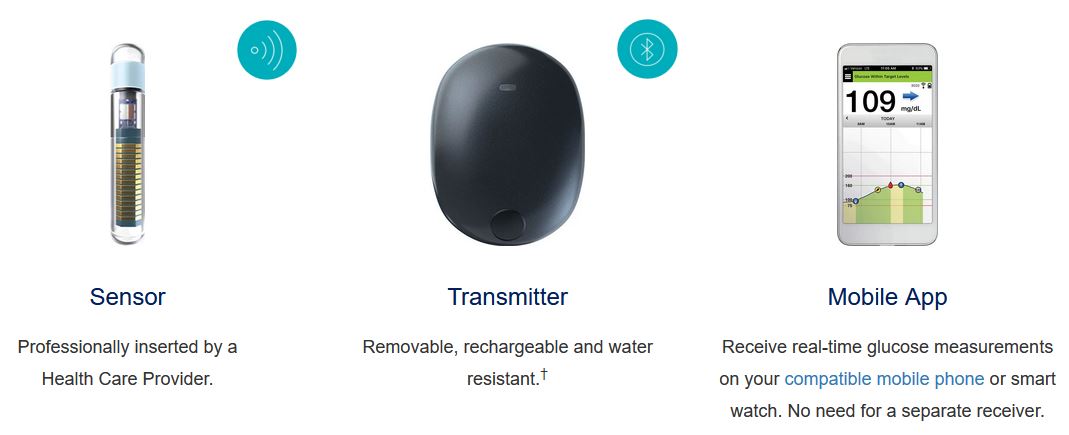
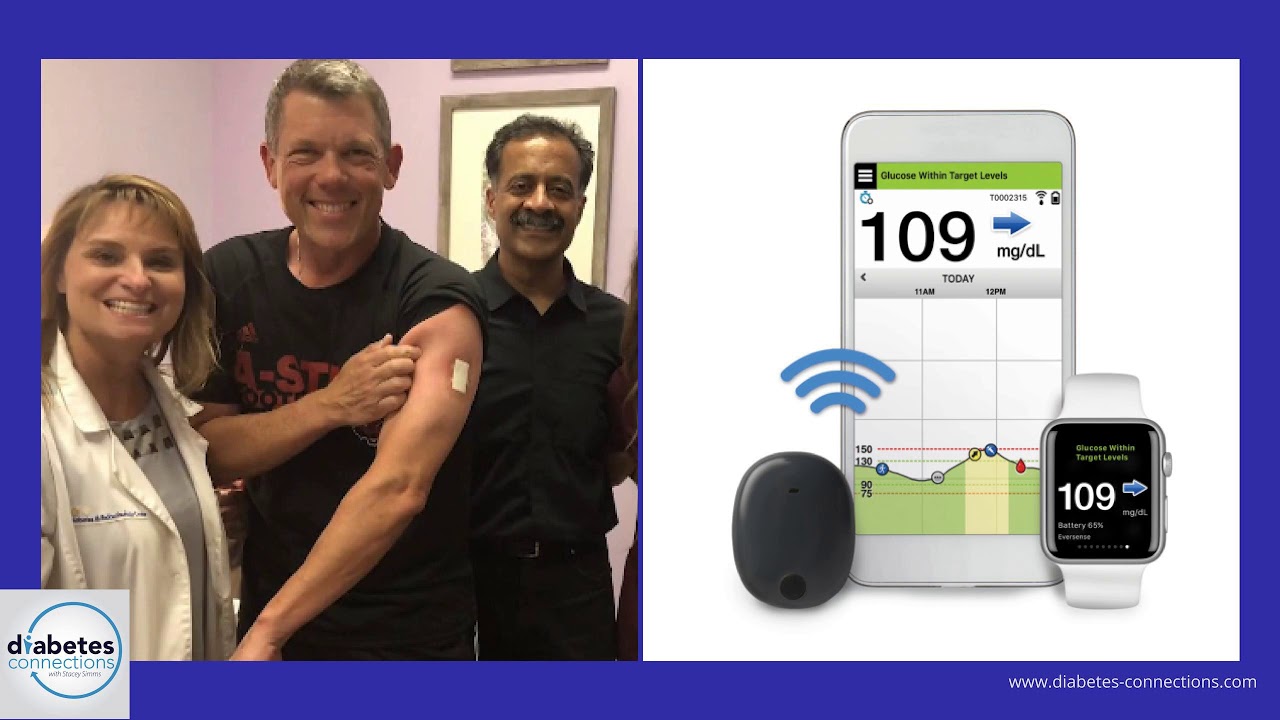
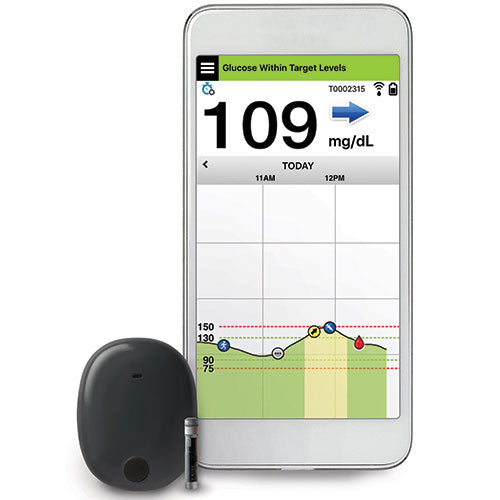
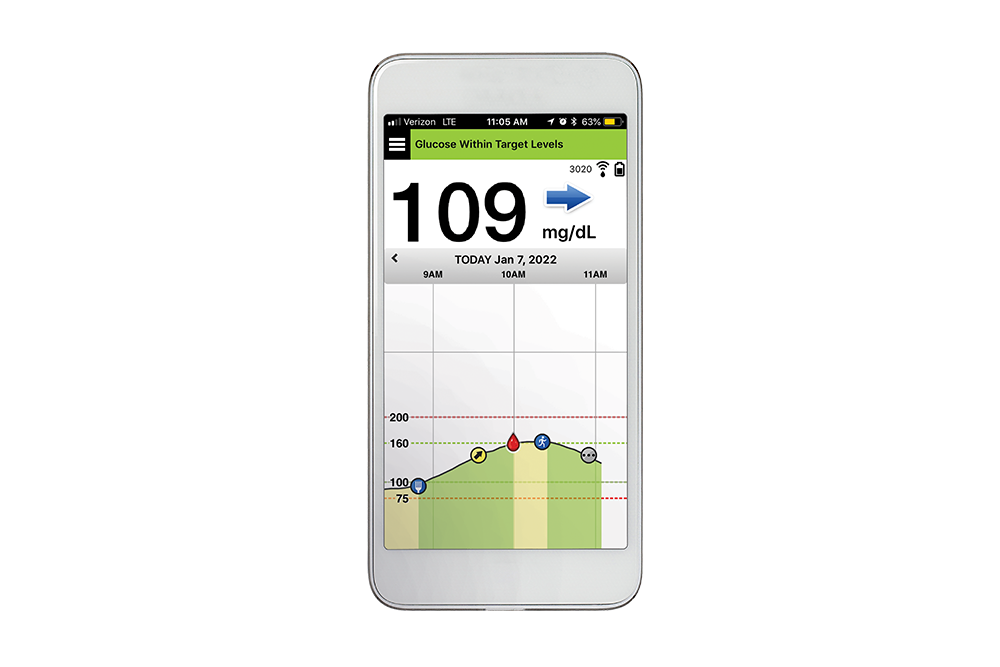
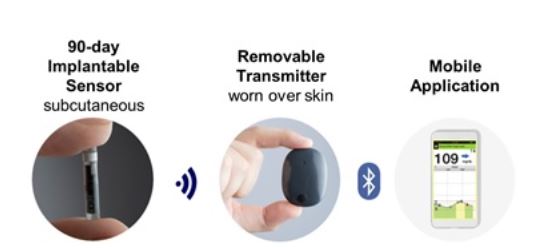
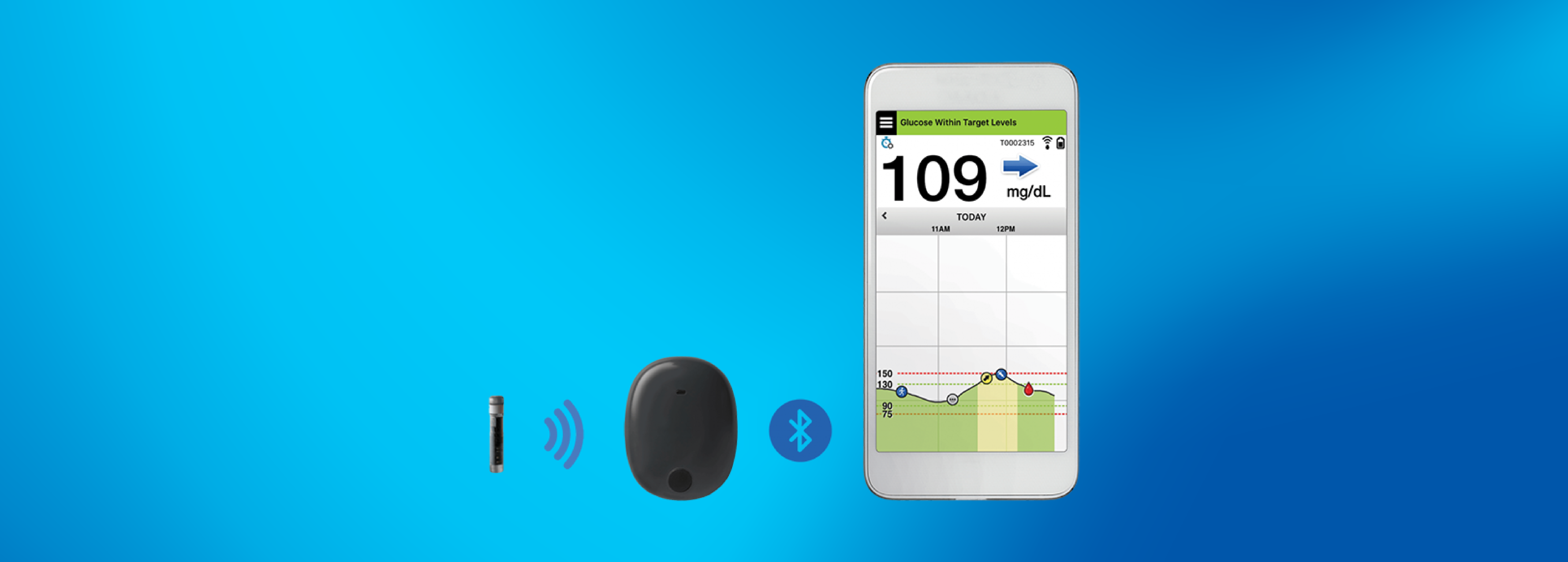
DiabetesMine.com - NEWS: Senseonics suspends U.S. sales of its Eversense implantable CGM. However, the company says it will (for now) continuing supporting existing customers, will still pursue R&D of its 180-day iteration,

Implantable glucose sensor featuring IDT sensing technology awarded CE Mark - Medical Design and Outsourcing
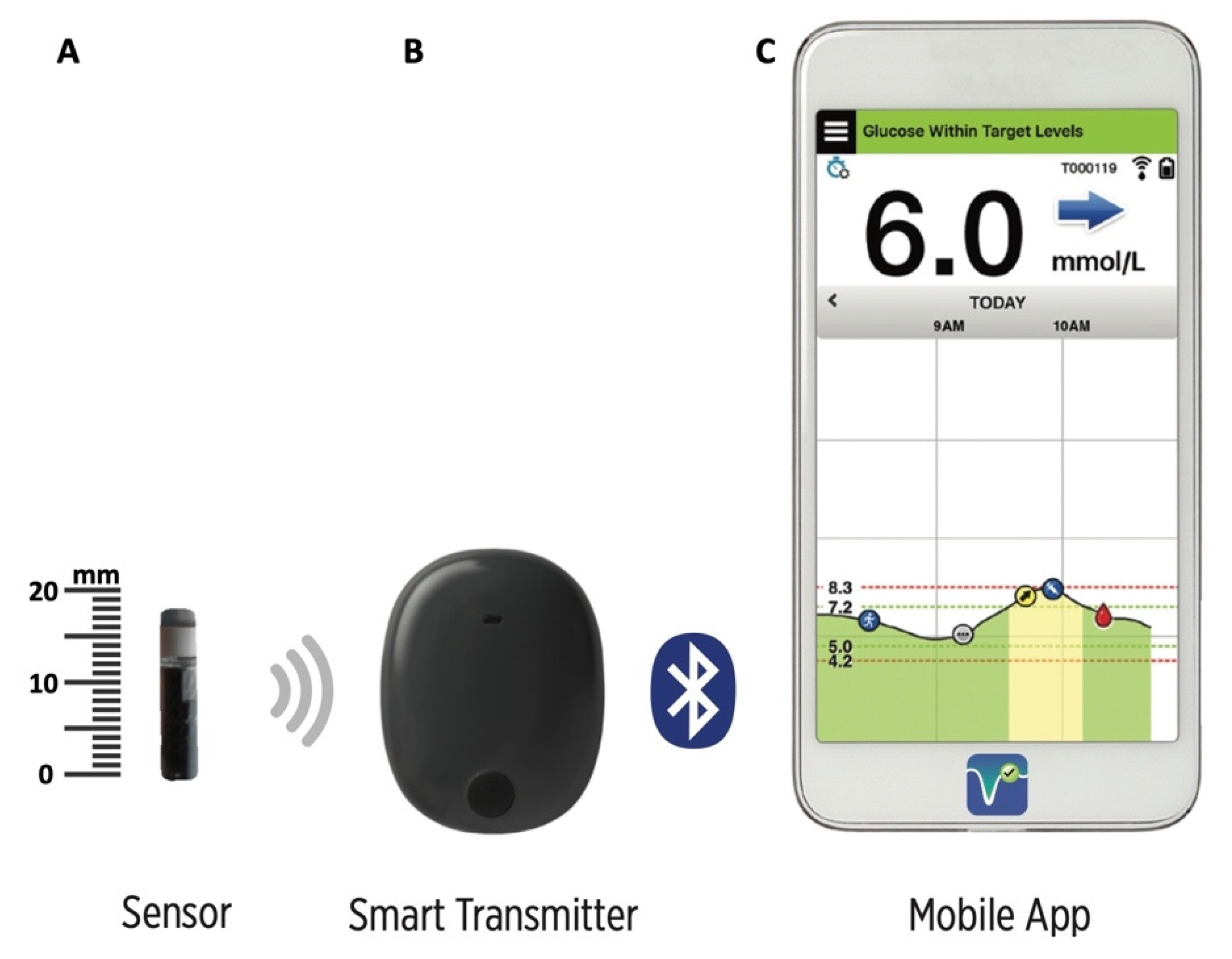
Animals | Free Full-Text | Clinical Use of a 180-Day Implantable Glucose Monitoring System in Dogs with Diabetes Mellitus: A Case Series

FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News